A nucleic acid is a polymer made up of many nucleotides. A nucleotide is generally made of;
i. Nitrogen-containing base; a heterocyclic molecule, containing a closed ring of atoms of which at least one is not a carbon atom and replaced with a nitrogen atom.
ii. Pentose sugar; meaning contains five carbon atoms in the sugar molecule.
iii. Phosphate Group; usually a phosphorus atom surrounded by a double bonded oxygen atom, single bonded to two oxygen negative ions and an oxygen atom.
Since the least complicated molecule is the phosphate group, it can easily be distinguished. However, for now, the distinguishing features for the nitrogen containing base will be the nitrogen atom. If the phosphate group is absent, the sugar-base combination is called a nucleoside.
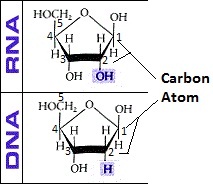
The most common two forms of nucleotides are Ribonucleotides which form the nucleic acid Ribonucleic acid (RNA) and Deoxyribonucleotides which form the polynucleotide or nucleic acid Deoxyribonucleic acid (DNA), the molecules responsible for coding the proteins in the cell. In ribonucleotides, the sugar is ribose. In deoxyribonucleotides, the sugar is deoxyribose.
The difference between the two is that the pentose sugar in DNA has one oxygen atom less in the hydroxyl group of Carbon atom 2. Also, since these two are the prime nucleic acids we shall be focusing on, their polymer structure is generally helical.
Another popular nucleotide is Adenosine Triphosphate (ATP) which is used as an energy currency in the cell. This will be clearer in later chapters.
Thus, nucleic acids are biopolymers composed of monomers called nucleotides. The term polynucleotide is a more accurate description of a single molecule of nucleic acid.
The nitrogenous bases of nucleotides belong to two families known as purines and pyrimidines.
In nucleotides, the base is joined to C-1 of the sugar and the phosphate group is attached to one of the other sugar carbons, usually C-5.
There are three phosphoryl groups; alpha(α), beta(β), and gama(γ) esterified to the C-5 hydroxyl group of the ribose. The linkage between ribose and the α-phosphoryl group is a phosphoester linkage because it includes a carbon and a phosphorus atom, whereas, the β- and γ-phosphoryl groups in ATP are connected by phosphoanhydride linkages that don’t involve carbon atoms. All Phosphoanhydride have considerable chemical potential energy, and ATP is no exception. This potential energy can be used directly in biochemical reactions.
In polynucleotides, the phosphate group of one nucleotide is covalently linked to the C-5 oxygen atom of the sugar of another nucleotide creating a second phosphoester linkage. The entire linkage between carbons of adjacent nucleotides is called a phosphodiester linkage, because it contains two phosphoester linkages.
Nucleic acids contain many nucleotide residues and are characterized by a backbone consisting of alternating sugars and phosphates. In DNA, the bases of two different polynucleotide strands interact to form a helical structure.
RNA contains ribose rather than deoxyribose, and is usually a single stranded polynucleotide. There are four kinds of RNA molecules: Messenger RNA (mRNA), transfer RNA (tRNA), Ribosomal RNA (rRNA), and a heterogeneous class of small RNAs that carry out a variety of different functions.