The most distinguishing feature of lipids are the hydrocarbon chain, with a carboxyl group (C=O) at the end. This is the basic structure of lipids and called fatty acid. There are usually between 16-18 carbon atoms in the hydrocarbon chain.
In the diagram, the fatty acid is seen attached to a glycerol molecule and a phosphate group. It is known as a phospholipid. The carboxyl end of the fatty acid is highly polar and therefore water soluble (hydrophilic meaning attracted to water). Hydrocarbon chain of the fatty acid is highly non-polar and therefore water insoluble (hydrophobic, which means scared of water).
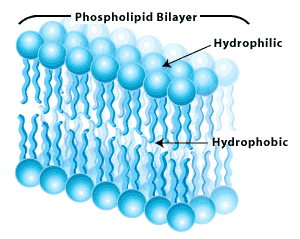
Image Credit: http://bioteach.ubc.ca/Bio-industry/Inex/
When fatty acids interact with water, the soluble carboxyl end dissolves and forms a layer with water, while the hydrocarbon tale remains outside the water surface. This quality is important in forming the bio membrane of cells which will become clearer below.
Also, another quality to remember is when all carbon atoms of the hydrocarbon chain in the fatty acid are joined by a single bond, the compound is said to be saturated, this means that every carbon atom has hydrogen atom on both sides. In unsaturated fatty acid, one or more carbon atoms form a double bond with another carbon atom. Therefore, it will not be able to hold a hydrogen atom and therefore said to be unsaturated. As you can observe from the first diagram, there are two hydrocarbon tails, one is saturated and the other unsaturated and where the carbon atom forms a double bond, there can be seen a kink in the tail. This kink provides a fluid quality to cells that allows it more flexibility in motility and structure and therefore is healthier than saturated fats that plague processed food.
Usually fatty acids are stored in the form of triglycerides– glycerol molecule + 3 fatty-acid tails. A glycerol molecule plus a fatty-acid tail is a glyceride molecule. The above diagram shows us a diglyceride consisting of two fatty-acids linked to a glycerol molecule. Triglycerides are insoluble in water and therefore group as fat droplets in the cytoplasm of the cell. When required, they can be broken down for use as energy.
Lipids provide an important form of energy storage, since they give more than twice as much energy as carbohydrates of the same mass. Also, as previously stated, they are the major components of cell membranes. Lastly, they play important roles in cell signaling. Example: steroid hormones, such as estrogen and testosterone are made of cholesterol and used in processing food and building nerve cells, apart from other metabolic functions.
Most lipids are not soluble in water, but they do dissolve in some organic solvents.
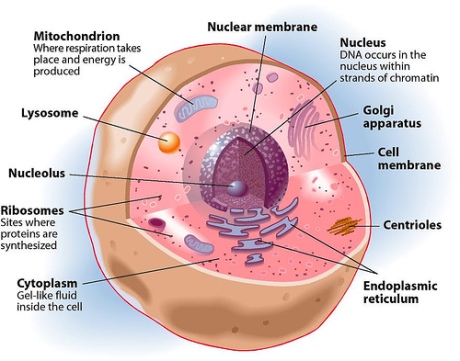
All cells are surrounded by a layer of membrane that separates their internal environment (cytoplasm) from the external environment (exoplasm or extracellular matrix). Additionally, the organelles in cells, are compartmentalized with the help of biomembranes, with similar chemical composition as that of the plasma membrane.
Cell Structure. Click on Image for Credit.
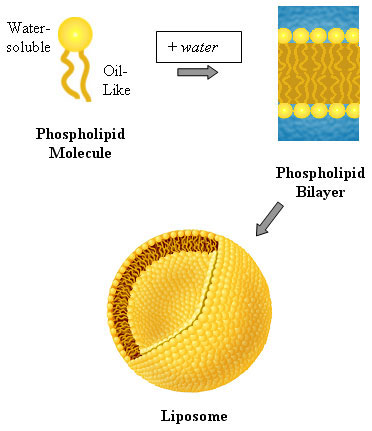
The Lipid Bilayer which is largely made of phospholipid; a glycerol molecule is joined to two fatty acid chains and the third site on the glycerol is linked to a hydrophilic phosphate group. Phospholipids are therefore amphipathic lipids, meaning they are partly water soluble and partly insoluble. This is because they have both hydrophobic (fatty acid tail) andhydrophilic (phosphate group) regions.
In order to understand the lipid bilayer structure of a cell, hypothetically imagine its development- If we try to dissolve one phospholipid in a water molecule, the hydrophilic head (which contains the phosphate group) will dissolve in the water, whilst the hydrophobic tail will remain outside the water. However, if two or more phospholipids get together, they will start forming a different structure; the heads will still dissolve in water, but the tails, rather than facing outwards, will face inwards towards each other shielding the water out.
This molecular character causes phospholipids to form a spherically bilayer structure called liposome. In it, all nonpolar tails in each phospholipid molecular layer is called a leaflet. Examples of phospholipids are phosphatidylcholine (the phosphate group is attached to a second small hydrophilic compound such as choline. See first image) and sphingomyelin.
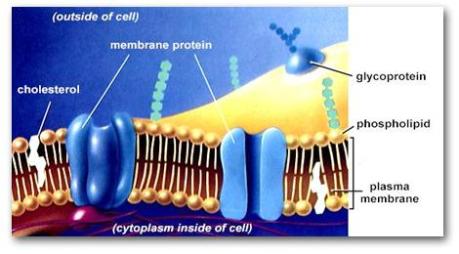
Because the lipid bilayer has a nonpolar hydrophobic core that is not easily permeable, biomembranes are interspersed or separated by proteins (referred to as membrane proteins) that allow certain molecules and ions to pass and so act as gateways or pathways towards and outwards the cell cytoplasm. The best analogy here is that of a concrete house interspersed with windows and doors to allow passage of people and air.
Structurally, the plasma membrane is referred to as having a Fluid Mosaic Model. This refers to the story of Prophet Moses (Mosaic) parting the sea, and crossing over. The protein gateway here provide that effect.
Cholesterol is another lipid molecule that is present in the biomembrane. It consists of four hydrocarbon rings (that are strongly hydrophobic) and a hydroxyl group (O-H) attached to one end and is weakly hydrophilic. This quality makes cholesterol amphipathic as well.
One of its main functions is that it can break down the tight connection of the biomembrane phospholipid bilayer making it more flexible for the passage of small molecules and ions. Because of this and the fact that phospholipids have the capacity of movement within the biomembrane (more on this later), it explains the presence of “fluid” in the Fluid Mosaic Model.
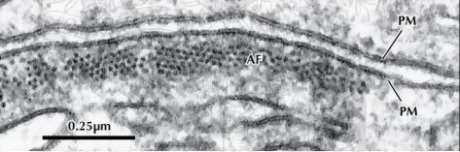
In the electron microscope, the cell membrane appears to have a trilaminar appearance (having three layers):
Two dark bands indicating the lipid bilayers of the hydrophobic tail and one bright band showing the intramembranous space between the bilayers. The hydrophilic core or polar head is not visible because it has dissolved in water.