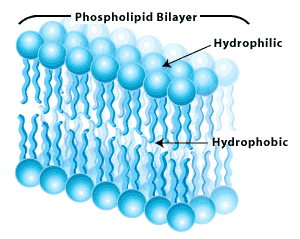
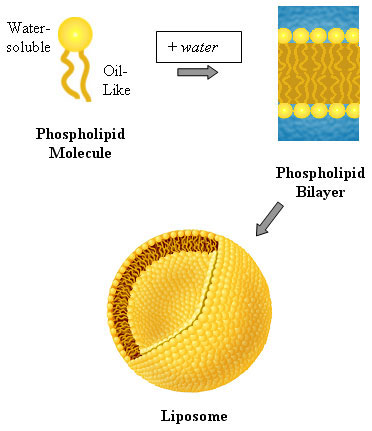
Among the important properties of water is its slight tendency to ionize. Pure water consists not only of H2O but also a low concentration of hydronium ions (H3O+) and an equal concentration of hydroxide ions (OH–):
2H2O (l) <–> H30 + (aq) + OH – (aq)
Hydronium ions are capable of donating a proton to another ion. Such proton donors are referred to as acids according to the Brønsted-Lowery concept of acids and bases.
Proton acceptors are called bases. Hydroxide ions can accept a proton and be converted back to water molecule. Therefore, what? That’s right. Hydroxide ions are bases.
The ionization of water can be analyzed quantitatively. Brace yourself for some math now. Don’t worry; it’s explained simply.
Since part of water dissociates in the water solution, we should note that the concentrations of reactants and products in a reaction will always reach equilibrium. In this case, the reactant is water H2O and the products are hydronium ions (H3O+) and hydroxide ions (OH–).
The ratio of these concentrations defines the equilibrium constant (Keq).
But first, we need to calculate the concentration and then its ratio. How can we calculate the concentration of water?
Formula for any concentration is M = n ÷ V, where M = concentration of solution, n = moles of substance, and V = volume of solution in litres (L).
So, to find concentration, we need to know the moles of the substance, in our case is water with one oxygen = 16gm and two hydrogen = 1 each (see periodic table), with a total of 16+1+1 = 18gms per mole of H2O.
The volume, let’s for simplicity take 1 litre. However, since the mole is in grams, we have to convert the units of litre into grams as well. Math 101 question: how many litres are there in a gram? That’s right, 12. No idiot, go back to math class. The correct answer is 1000gms. “You Baboon! You Dracula!”,(our economics teacher).
Okay, now that we have two similar units, we must apply the formula:
(1000g of H2O per L) / (18g of H2O per mole) = 55.55.
The concentration of a solution is usually given in moles per litre (mol L-1 OR mol/L) since the grams cancel each other out. This is also known as molarity.
Concentration, or molarity, is given the symbol M. Example; a short way to write that the concentration of a solution of hydrochloric acid is 0.01 mol/L is to write [HCl] = 0.01M. Got it?
So the molarity of water is 55.5 mol/L or mol L-1 or M.
By the way, usually, the square brackets around the substance indicate concentration. So if we write [H2O] = 55.5M, we’re trying to say that the concentration or M of water is…you get it.
Now that we have the concentration, we have to bear in mind (and as already stated) that the two products of ionization of water dissociate into equal concentrations.
Assuming that the concentrations of hydronium and hydroxide ions are very low relative to the concentration of un-ionized water, then the formula is:
Keq ×55.5 M = [H3O+] [OH–]
The equilibrium constant for the ionization of water is 1.8 × 10-16M at 25°C. Substituting this value in the above equation gives:
1.8 × 10-16 M × 55.5 M = [H3O+] [OH–]
Therefore, 1.0 × 10-14 M2 = [H3O+][OH–]
[H3O+][OH–], called the ion product for water, is a constant designated Kw, and hence has a value of 1.0 × 10-14 M2.
Because water is electrically neutral, its ionization produces an equal number of hydronium ions and hydroxide ions [H3O+] = [OH–]. In the case of pure water, the above equation can therefore be rewritten as:
Kw = [H3O+] 2 = 1.0 × 10-14 M2.
Taking the square root of the terms gives; [H3O+] = 1.0 × 10-7 M.
Since [H3O+] = [OH–], the ionization of pure water produces 10-7 M of H+ and 10-7 M of OH–.
One interesting point to note is that the hydronium ions are so ionic that they immediately donate a proton (H+)in a reaction, becoming H2O + H+. Therefore, when we refer to hydronium ions, we are more specifically talking about it’s capacity to donate a proton and therefore, can neglect the H2O and simply discuss the H+ to the point where the H3O+ in the entire equations above can be replaced simply with H+ (as we did only in the end, “pure water produces 10-7 M of H+and 10-7 M of OH–”).
Pure water and aqueous solutions containing equal concentrations of H+ and OH– are said to be neutral. Of course not all aqueous solutions have equal concentrations of H+ and OH–.
When an acid is dissolved in water, H+ increases and the solution is described as acidic solution.
Note that when an acid dissolves in water, the concentration of protons increases, conversely, the concentration of hydroxide ions decreases. This is because the ion product constant for water (Kw) in unchanged (i.e., constant) and the product of the concentrations of H+ and OH– is always1.0 × 10-14 M2.
So evidently, dissolving a base in water decreases [H+] and increases [OH–] above 1.0 × 10-7 M, producing a basic or alkaline solution.